LeakyGutMicrobiomeLabs.jpg
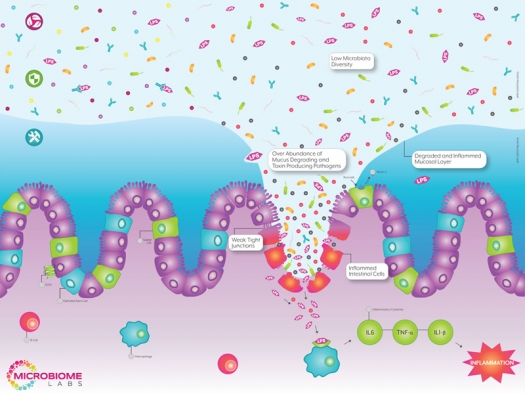
Illustration courtesy of Microbiome Labs
Leaky gut, or intestinal permeability, is a condition in which bacteria and their toxins are able to leak through physical holes in the intestinal wall and migrate directly into the bloodstream. In fact, some research suggests that leaky gut may very well be ground zero for many chronic diseases. In 2015, a review article in Frontiers of Immunology found that increased intestinal permeability, in addition to modern lifestyle factors, was capable of triggering a low-grade inflammatory state through the influx of bacterial endotoxins.1
The most common endotoxin is lipopolysaccharide (LPS), a major component of the outer cell membrane of gram-negative bacteria. When found in the intestinal lumen, this compound is mostly harmless. However, once released into the bloodstream, LPS can trigger an acute inflammatory reaction.2 While it receives little recognition for the damage that it causes, LPS has been used extensively in the lab to induce a number of chronic diseases, including Alzheimer’s, multiple sclerosis, inflammatory bowel disease, cardiovascular disease, diabetes, obesity, hypogonadism, autoimmunity, and mood disorders like autism, anxiety, and depression.3
The findings of this 2015 study suggest that leaky gut plays a “pivotal and perhaps even causal role in the development of low-grade inflammation and its related diseases.”1
Causes of leaky gut
Some of the basic lifestyle factors that contribute to leaky gut include chronic alcohol consumption, chronic smoking, intense exercise, lack of sleep, and overuse of medications like antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs), and proton-pump inhibitors (PPIs).4 However, one of the major contributors to overall gut dysfunction is stress.1, 5 Whether it’s mental, physical, or emotional, too much stress can have detrimental effects on your health.
In 2011, researchers in Germany found that exposure to stress was responsible for alterations in gut motility, an increase in intestinal permeability, and negative effects on the intestinal microbiota, as well as the regenerative capacity of the gut mucosa. These findings suggest that stress management could be particularly effective in the treatment of digestive and autoimmune conditions. Additionally, researchers concluded that “probiotics may profoundly affect the gut-brain axis and attenuate the development of stress-induced disorders in the upper and lower GI tract.”6
How to stop the leak
Some basic lifestyle choices, like managing stress, limiting alcohol, and getting adequate sleep, can all significantly reduce the risk for developing leaky gut. However, since most people already have leaky gut to some degree, there are also some promising interventions.
Increase secretory IgA levels
One way to prevent LPS from seeping into the bloodstream is to increase secretory IgA levels. Secretory immunoglobulin A (sIgA) is the first line of defense against liberated LPS in the intestines because it has the unique capability of binding and neutralizing LPS directly in the gut. Nutrients that can increase sIgA levels include omega fatty acids, glutathione, glutamine, glycine, vitamin C, zinc, and colostrum powder.
Increase mucin production
Another way to strengthen the intestinal lining is to thicken the protective mucosal barrier known as the intestinal mucosal layer. This layer creates a thick physical buffer between the intestines and the outside world and is made up primarily of a thick protein called mucin. Nutrients that can increase mucin production include threonine, serine, protein, and cysteine.
Modulate the microbiome
An all-encompassing approach would be to utilize spore-based probiotics that are capable of modulating the intestinal microbiome, up-regulating sIgA levels, and thickening the intestinal mucosa. The major concern with most probiotics is the issue of survivability. Most probiotic strains are not designed to withstand stomach acid, bile salts, the lack of oxygen, or the competitive environment in the large intestine. For this reason, most probiotics fail to make any lasting changes to the gut microbiome or have any protective effects on the host. However, spore-based probiotics are uniquely designed to survive digestion and compete in the large intestine with ease. For these reasons, spore-based probiotics are some of the most promising leaky gut treatments on the market today.
A recent study at the University of North Texas found that just 30 days of supplementation with a spore-based probiotic demonstrated a 45% reduction in serum LPS levels following a high-fat meal. Interestingly, the placebo treatment group showed a 28% increase in serum LPS levels after 30 days of no treatment, suggesting that leaky gut may be a progressive condition that requires continuous intervention.7
To date, bacterial spores are the only probiotic strains that have been shown to reduce leaky gut and may be one of the most effective treatments for leaky gut and its associated chronic diseases.
For more information, go to Microbiome Labs.
References
- De Punder K, Pruimboom L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front Immunol. 2015;15(6):223.
- Nakarai H, Yamashita A, Nagayasu S, et al. Adipocyte-macrophage interaction may mediate LPS-induced low-grade inflammation: potential link with metabolic complications. Innate Immunity. 2012;18:164-70.
- Qin L, Wu X, Block ML, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453-62.
- Yoshikawa K, Kurihara C, Furuhashi H, et al. Psychological stress exacerbates NSAID-induced small bowel injury by inducing changes in intestinal microbiota and permeability via glucocorticoid receptor signaling. J Gastroenterol. 2017;52(1):61-71
- Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiologic stress. Am J Physiol Gastrointest Liver Physiol. 2017;312(6):G559-571.
- Konturek PC, Brzozowski T, Konturek SJ. Stress and The Gut: Pathophysiology, Clinical Consequences, Diagnostic Approach and Treatment Options. J Physiol Pharm. 2011;62(6):591-599.
- McKarlin BK, Henning AL, Bowman EM, et al. Oral spore-based probiotic supplementation was associated with reduced incidence of post-prandial dietary endotoxin, triglycerides, and disease risk biomarkers. 2017. Manuscript submitted for publication.


